SSR Inst. Int. J. Life.
Sci., 5(4): 2355-2360, July 2019
Comparison of
Real time PCR and Conventional PCR for Detection of HLA-B27 in Suspected
Ankylosing Spondylitis Patients
Jaspreet Kaur1, Jaswinder
Singh2*
1Senior Research Associate,
Department of Biochemistry, Shri Ram Murti Smarak Institute of Medical
Sciences, Bareilly, U.P., India
2Associate Professor,
Department of Forensic Medicine and Toxicology, Shri Ram Murti Smarak Institute
of Medical Sciences, Bareilly, U.P., India
*Address for Correspondence: Dr. Jaswinder
Singh, Associate Professor, Department of Forensic Medicine and Toxicology, Shri
Ram Murti Smarak Institute of Medical Sciences, Bareilly-243202, U.P., India
E-mail: drjasvinder2013@gmail.com
ABSTRACT-
Background- Human leukocyte antigen B27 (HLA-B27) is a major
histocompatibility complex (MHC) class 1 molecule that is strongly associated
with the chronic inflammatory disease ankylosing spondylitis (AS). The aim of the present study
was to evaluate the utility of real time PCR over conventional PCR to find out
the involvement of HLA-B27 in relation to age and sex in symptomatic suspected
patients.
Methods-
The present cross-sectional study was conducted on the 320 suspected AS
patients. Blood samples from patients were processed for PCR and real time PCR
using HLA-B27 as a gene target.
Results-
Out of 320 samples, 153 (48%) and 163 (51%) patients are found to be HLA-B27
positive in PCR and real time PCR respectively. Out of 153 and 163 positive
samples, the positivity rate is maximum i.e. 59% and 61% in the patients of
16-30 years of age in PCR and real time PCR respectively. Furthermore, in terms
of gender wise variation, 115 (75%) and 126 (77%) are male and remaining 38
(25%) and 37 (23%) are female in PCR and real time PCR respectively. Our data
revealed sensitivity and specificity of real time PCR for HLA-B27 positive
cases are 100% (95% CI: 89.11 - 100% with p-value 0.002) and 94% (95% CI: 73.05
- 91.21% with p-value 0.012) respectively. The PPV is 93.87% (95% CI: 73.01 -
105.36% with p-value 0.015) and NPV is 100% (95% CI: 83.19 - 111.03% with
p-value 0.101).
Conclusion-
It could be concluded that the real time PCR test is a fast, accurate and
sensitive method for the diagnosis of AS.
Key Words:
Ankylosing spondylitis, HLA-B27, PCR, Real time PCR, Spondyloarthropathies
INTRODUCTION- The human leukocyte antigen system is a gene complex encoding the major histocompatibility
complex proteins in humans. These cell-surface proteins are responsible for the regulation of
the immune system. Although
HLA molecules are best known for their role in transplantation, certain HLA
molecules are associated with specific diseases. HLA-B27 (subtypes
B*2701-2759) is an MHC class I surface antigen encoded by B locus in the MHC on the short (p) arm of chromosome no.
6 and presents antigenic peptides
(derived from self and non-self antigens) to T
cells [1]. MHC class I molecules
are cell-surface glycoproteins that are expressed on most nucleated human cells
and platelets. The presence of HLA-B27 antigen is strongly associated with a
number of rheumatic diseases, including AS, Reiter’s syndrome, acute
anterior uveitis, and inflammatory bowel disease (IBD) [2]. AS is a
chronic inflammatory disorder of unknown cause that primarily affects the axial
skeleton; peripheral joints and extra-articular structures may also be
involved. AS affects men and women; the male to female prevalence is
approximately 3:1. AS is a type of spondyloarthropathy (SpA) that often starts
in the late teens and early 20s but may also present earlier in childhood or at
an older age [3]. Today the exact cause of AS is not known but the
gene for HLA-B27 is present in 90% of all patients with AS and 50 - 75% of
patients with other SpA diseases and therefore it is considered as a
contributing factor. Studies on mice and rats using HLA-B27 as a transgene
developed ankylosing enthesopathy and rats having high copy number of HLAB*2705
developed axial and peripheral arthritis, gut inflammation, and lesions on the
skin showed strong evidence of the involvement of HLA-B27 in AS [4,5].
The association with B27 is independent of disease severity. AS shares many
features with several other arthritis conditions, such as psoriatic arthritis,
reactive arthritis, and arthritis associated with Crohn’s disease and
ulcerative colitis. Each of these arthritic
conditions can cause disease and inflammation in the spine, other joints, eyes,
skin, mouth, and various organs [6]. Mostly
HLA-B27 testing is performed with surface antigen tests. Two commercially
available antibodies are commonly used. The cross-reactivity of these
antibodies can compromise the accuracy of the results generated in the antigen
assays [7]. Although the conventional PCR technology is a rapid and
sensitive method for the diagnosis of AS [8,9], the main drawback is
that it is time taking and visualization is achieved only after electrophoresis
and staining with Ethidium bromide (a fluorescent DNA intercalating dye) and
viewing the gel under UV light. The aim of the present study is to evaluate the
utility of fluorogenic real time qualitative PCR over conventional PCR to
detect the presence of the B*27 genotype by amplifying a region between primer
sets and a specific dual labeled hydrolysis probe that recognize only B*27
specific sequences. The advantages of this method are that primers and probes
provide specificity to the reaction products and allow immediate visualization.
The analytical capacity of this technique is much greater than the allele specific
PCR and allows for near complete automation.
MATERIALS
AND METHODS-
Study specimens- The present cross-sectional study was conducted on the 320
suspected AS patients attending the out and indoor patient departments
(Medicine, Paediatrics, Orthopedics, and General Surgery) of Shri Ram Murti
Smarak Institute of Medical Sciences (SRMS IMS), Bhojipura, Bareilly, India
from Aug 2015 to Apr 2019. Two milliliters of blood was collected in a BD
Vacutainer® (Cat. No. 367841) from each patient and transported at 4°C to the
Central Research Laboratory of the Department of Biochemistry for further
processing. Written consent was obtained from each patient for the present
study. The clinical specimens used in this study were collected from patients
of all age group and both sexes with suspected AS on the basis of clinical
criteria or to rule out AS. The clinical criteria used was that the patients
having low back pain and stiffness for more than 3 months, limitation of motion
of the lumbar spine in both the sagittal and frontal planes and limitation of
chest expansion relative to normal values corrected for age and sex. All procedures performed in studies involving human
participants were in accordance with the ethical standards of the institute.
Nucleic
Acid extraction- DNA was isolated from EDTA whole blood
samples from 320 consecutive samples sent for HLA-B27 testing at our laboratory using QIAamp DNA Blood Mini Kit (Qiagen Inc., Valencia,
CA, USA) (Cat no. 51304) as per manufacturer’s instructions. The final DNA concentration was approximately 20 ng/µl
and was quantified by absorbance at A260 for dilution experiments.
A 5μl aliquot of DNA was used for each PCR reaction, and therefore
approximately 100 ng of each sample was used, although there was considerable
variation.
Conventional
PCR- The PCR was performed in a total volume of 25
μl containing 2.5 μl of 10x reaction buffer HiBufferA with 2.5 mM
MgCl2, 2.5 μl of 2.5 mM dNTP’s, 1U HotStart Taq DNA polymerase [10].
The primer concentration was 1µl (1µmol/µl) in total, 0.5 µl of the HLA-B27
specific primers (5' primer, B27ex294F: 5'- CTACGTGGACGACACGCT-3'; 3' primer,
B27ex2199RC: 5'-AGTCTGTGCCTTGG CCTTGC-3') and 0.5µl of the human growth hormone
(HGH) specific primers HGHI (5'-CAGTGCCTTCCCAACCATTCCCTTA-3') and HGH2
(5'-ATCCACTCACGGATTTCTGTTGTGTTTC-3'). One cycle of denaturation at 96°C for 4
minutes was followed by 40 cycles at 96°C for 30 seconds, 58°C for 30 seconds,
72°C for 30 seconds followed by a final extension at 72°C for 5 minutes.
Amplified products were visualized under UV light after electrophoresis on a 2%
agarose gel and staining with ethidium bromide. Samples which had amplified
products measuring 141 bp band for HLA-B27 were considered positive. Internal
control produces a 437 bp band, which must be present to validate the assay.
Real
time PCR- Real time PCR amplification for HLA-B27 was
performed using the 3B HLA-B27 detection kit (3B BlackBio Biotech India Ltd)
(Cat no. 3B247), in accordance with the manufacturer's protocol in a CFX96TM
real time system (BIO-RAD). This
qualitative real time PCR test kit was based on the amplification of the
allelic gene region by primer and probes specific for HLA-B27 with a
sensitivity of ≥10 copies in every microliter of sample. The probe
contains a fluorescent dye molecule on its 5’ end and a quencher molecule on
its 3’ end. The probe hybridizes with one of the chains of the amplified
fragment. During the synthesis of a complementary chain, Taq DNA polymerase,
which possesses 5’-3’ exonuclease activity cleaves the probe. As a result, the
fluorescent dye and quencher dye were separated, and the total fluorescence of
reaction volume increases in direct proportion to the number of amplicon copies
synthesized during PCR. In this kit there were two independent reactions
running in parallel in each tube: the first detects HLA-B27 (FAM channel at 520
nm) and second detects internal control (HEX channel at 556 nm), which allows
excluding unreliable results. The cycling conditions were 10 minutes at 95°C
and 40 cycles of 15 seconds at 95°C, 60 seconds at 60°C (single acquisition of
fluorescence signals). The fluorescent signal was measured in each cycle of
reaction, and the cycle threshold value (Ct) was determined from the
obtained curve. The Ct was proportional to the initial number of DNA
copies in a sample and its value allows qualitative comparisons of analyzed and
control samples.
Quality control- Reagents
were aliquoted and used only once for every reaction. Sterile microfuge tubes
and PCR tubes were used for the PCR assay. Reagent preparation, DNA extraction,
amplification, and detection were performed in separate rooms to avoid
cross-contamination of amplicons. Positive control was included in each test
and distilled water was included as a negative control.
Statistical
analysis- Data were analyzed using SPSS 18.0 (Statistical
Package for the Social Sciences, Chicago, IL, USA) for Windows. Performance of
PCR-based NAAT (Nucleic Acid Amplification Test) was reported in terms of
sensitivity and specificity at 95% confidence interval along with their
p-values. p<0.05 was considered statistically
significant.
RESULTS- DNA was extracted from 320 samples and
amplification was done using allele-specific conventional PCR [10].
Briefly, conventional PCR amplifies a 141bp band from exon 2 of the HLA-B27
family. Internal control primers specific for the HGH produces a 437bp band
which must be present to validate the assay.
In real time PCR, the fluorescent signal was generated from an
oligonucleotide probe (fluorescent reporter dye probe) specifically used for
HLA-B27 gene sequence. The appearance of amplification signal in real time PCR
indicates the presence of HLA-B27 gene along with amplification signal of
internal control, which was used for the validation of the result as it was
present in the normal population as well as in patients (Fig. 1).
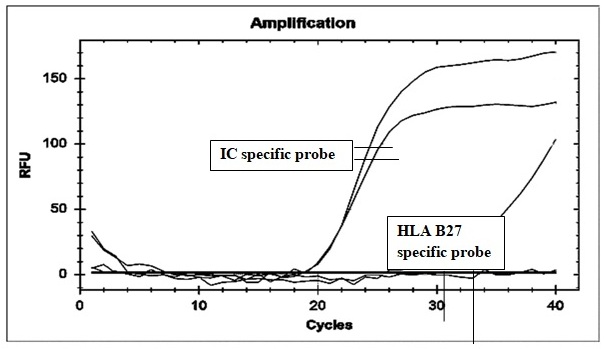
Fig.
1: A curve for each
well is constructed for internal control and HLA-B27 specific probes with cycle
number (x-axis) vs. relative fluorescence unit (y-axis). The horizontal line is
set manually and results calculated for each well. Positivity is expressed as a
curve for internal control and HLA-B27 for those wells, where fluorescence
levels are above the threshold
Out
of 320 samples collected for the proposed study, 153 (48%) patients were found
to be HLA-B27 positive while remaining 167 (52%) were negative in PCR. In real
time PCR 163 (51%) patients were found to be HLA-B27 positive, while remaining
157 (49%) were negative. When samples were analyzed in terms of age groups, it
was found that out of 153 PCR positive samples, the positivity rate was maximum
i.e. 59% and 57% in the patients of 16 - 30 years and 0-15 years of age
respectively. However, in the age group of 31 - 50 years and above, the
positivity rate was 37% and 30% respectively (Table 1). Out of 163 real time
PCR positive samples, the positivity rate was maximum i.e. 61% and 60% in the
patients of 16 - 30 years and 0-15 years of age respectively. However, in the
age group of 31 - 50 years and above, the positivity rate was 41% and 34%
respectively, which were comparatively less (Table 1).
Table 1: Age wise distribution of
positivity rate of the presence of HLA-B27
|
S.
No. |
Age
group (years) |
No. of HLA-B27
positive patients in PCR/ No. of patients of that particular age group |
Positivity rate (%) in PCR |
No. of HLA-B27
positive patients in real time PCR/ No. of
patients of that particular age group |
Positivity rate
(%) in real time PCR |
|
1. |
0-15 |
17/30 |
57 |
18/30 |
60 |
|
2. |
16-30 |
86/145 |
59 |
89/145 |
61 |
|
3. |
31-50 |
34/92 |
37 |
38/92 |
41 |
|
4. |
Above
51 |
16/53 |
30 |
18/53 |
34 |
|
Total |
153/320 |
48 |
163/320 |
51 |
|
Furthermore, in terms of gender wise variation, 115 (75%) and 126
(77%) were male and remaining 38 (25%) and 37 (23%) were female in PCR and real
time PCR respectively (Table 2).
Table
2: Sex wise distribution of HLA-B27
|
S. No. |
Sex |
No. of PCR positive patients
(153) |
Positivity rate (%) |
No. of real time PCR positive
patients (163) |
Positivity rate (%) |
|
1.
|
Male |
115/153 |
75 |
126/163 |
77 |
|
2.
|
Female |
38/153 |
25 |
37/163 |
23 |
Our
data revealed sensitivity and specificity of real time PCR for HLA-B27 positive
cases were 100% (95% CI: 89.11 - 100% with p-value 0.002) and 94% (95% CI:
73.05 - 91.21% with p-value 0.012) respectively. The positive predictive value
(PPV) was 93.87% (95% CI: 73.01-105.36% with p-value 0.015) and negative
predictive value (NPV) was 100% (95% CI: 83.19-111.03% with p-value 0.101).
DISCUSSION-
In the present study, the positivity rate of HLA-B27
in different age groups and gender wise distribution of the HLA-B27 in patients
using conventional PCR and real time PCR approach were determined. Out of 320
samples collected for the proposed study, 153 (48%) and 163 (51%) patients were
found to be HLA-B27 positive in PCR and real time PCR respectively. This result
showed that the positivity rate of HLA-B27 was low in PCR in comparison
with real time PCR. A negative PCR result in
10 samples was indicated by the lack of amplification with the control primers.
Amplification failures may be explained by variations in small sample volume
when samples are inadequately mixed.
Many
factors influence the specificity of the PCR like GC content of the primer, Mg2+
ion concentration, ratio of primer to target, reaction buffer, and polymerase
enzyme concentration. Sometimes samples can be contaminated with previously
amplified DNA. Contamination of reagents can be minimized by preparing
solutions in separate room, which have not been exposed to amplified products,
aliquoting reagents for single use only, and using dedicated consumables and
equipment.
When samples were analyzed in terms of age and gender, it was
found that maximum positivity rate in 16 - 30 years followed by 0 - 15 years of
age with approximately 3:1 male to female ratio. In
conclusion, positive HLA-B27 status and male gender are associated with earlier
age at disease onset. These results were concordance with the previous
results [11].
It has been well established that 90 - 95% of patients with AS
have HLA-B27 positive, while 5–9% of the general population with AS may have
other contributory factors [12]. In our study NPV was found to be
100% means that 157 negative patients were true negatives and may have other
arthritis conditions.
Although
the pathogenesis of AS is incompletely understood but is almost certainly
immune mediated. The dramatic response
of all aspects of the disease to therapeutic blockade of tumor necrosis factor
α (TNF α) indicates that this cytokine plays a key role in the
immunopathogenesis of AS [13]. No specific event or exogenous agent
that triggers the onset of disease has been identified, although overlapping
features with reactive arthritis and IBD suggest that enteric bacteria
particularly Klebsiella pneumonae may
play a role. Elevated serum titers of IgA to certain enteric bacteria are
common in AS patients, but no role for these antibodies in the pathogenesis of
AS has been identified [14,15].
It was important to establish the diagnosis of early AS before the
development of irreversible deformity. Modified New York criteria are widely
used as gold standard for diagnosis. The clinical symptoms consist of a history
of inflammatory back pain, limited motion of the lumbar spine in the saggital
and frontal planes, limited chest expansion, relative to standard values for
age and sex and definite radiographic sacroiliitis. The presence of
radiographic sacroiliitis plus any one of the other three criteria were
sufficient for a diagnosis of definite AS. The presence of HLA-B27 was neither
necessary nor sufficient for the diagnosis, but the HLA-B27 test can be helpful
in patients with suggestive clinical findings who have not yet developed
radiographic sacroiliitis. Moreover, the absence of HLA-B27 in a typical case
of AS significantly increases the probability of coexistent IBD. Serological
techniques such as flow cytometry, microlymphocytotoxicity (MLCT) and ELISA for
testing HLA-B27 may give false negative results if HLA-B27 is down regulated or
‘‘masked’’. Flow cytometry is rapid and relatively inexpensive but the cross
reactivity of HLA-B27 with HLA-B7 decrease its specificity [16].
Identification of HLA-B27 by PCR supports the diagnosis of AS in symptomatic
individuals and negative results exclude the diagnosis [8]. Real
time PCR is a rapid and sensitive method as compared to other diagnostic
methods for the detection of HLA-B27 [17]. The main advantage was
its rapidity and the possibility of automation. Moreover, identification of
HLA-B27 will lead to a better understanding and treatment of the entire group
of diseases collectively known as spondyloarthropathies.
CONCLUSIONS- Real time PCR detection of HLA-B27
is found to be superior to that of conventional PCR; the latter yielded false
negative results. It is a fast, accurate, automated, and sensitive method for
the diagnosis of AS. The main advantage of this method is that the fluorescent
probe provides specificity to the reaction product and allows immediate
visualization.
Real
time PCR superiority over other diagnostic techniques in diagnosis of AS makes
it favorable in early diagnosis of disease and hence helps in early treatment
of patients and allays their suffering. Hence Real Time PCR must be used for
the diagnosis of HLA-B27.
ACKNOWLEDGEMENTS- The authors thank SRMS IMS Trust, Bareilly for providing the platform for such molecular diagnostic study in our laboratory.
CONTRIBUTION OF AUTHORS
Research concept- Dr. Jaspreet Kaur
Research design- Dr. Jaspreet Kaur
Supervision-
Dr. Jaspreet Kaur
Materials-
Dr. Jaspreet Kaur
Data collection-
Dr. Jaspreet Kaur
Data analysis and Interpretation- Dr. Jaspreet
Kaur
Literature search- Dr. Jaspreet
Kaur
Writing article-
Dr. Jaspreet Kaur
Critical review
Dr. Jaswinder Singh
Article editing-
Dr. Jaswinder Singh
Final approval- Dr. Jaspreet Kaur, Dr. Jaswinder
Singh
REFERENCES
1.
Rudwaleit M. New approaches
to diagnosis and classification of axial and peripheral spondylarthritis. Curr.
Opin. Rheumatol., 2010; 22: 375–80.
2.
Lopez-Larrea C,
Gonzalez-Roces S, Alvarez V. HLA-B27 structure, function, and disease
association. Curr. Opin. Rheumatol., 1996; 8: 296–308.
3.
Khan MA. Update on
spondyloarthropathies. Ann. Intern.
Med., 2002; 136: 896–907.
4.
Boyle LH, Goodall JC, Opat
SS, Gaston JS. The recognition of HLA-B27 by human CD4(+) lymphocytes. J.
Immunol., 2001; 167: 2619–24.
5.
Akkoc N, Khan MA.
Etiopathogenic role of HLA-B27 alleles in ankylosing spondylitis. APLAR J.
Rheumatol., 2005; 8: 146–53.
6.
Brionez TF, Reveille JD. The
contribution of genes outside the major histocompatibility complex to
susceptibility to ankylosing spondylitis. Curr. Opin. Rheumatol., 2008; 20(4):
384–91.
7.
Levering WH, Wind H, Sintnicolaas K,
Hooijkaas H, Gratama JW. Flow cytometric HLA-B27 screening: cross-reactivity
patterns of commercially available anti-HLA-B27 monoclonal antibodies with
other HLA-B antigens. Cytometry B. Clin. Cytom., 2003; 54(1): 28–38.
8.
Sharma N, Sharma V, Masood T, Nautiyal
SC, Sailwal S, et al. Usage of Conventional PCR Technology for the Detection of
HLA-B27 Allele: A Significant Molecular Marker of Ankylosing Spondylitis. Ind.
J. Clin. Biochem., 2013; 28(2): 189–92.
9.
Seipp
MT, Erali M, Wies RL, Wittwer C. HLA-B27 Typing: Evaluation of an
Allele-Specific PCR Melting Assay and Two Flow Cytometric Antigen Assays. Cytometry B. Clin. Cytom., 2005; 63: 10–15.
10. Sayer
DC, Cassell HS, Christiansen FT. HLA-B*27 typing by sequence specific
amplification without DNA extraction. Mol.
Pathol., 1999; 52: 300–01.
11. Xiong
J, Chen J, Tu J, Ye W, Zhang Z, Liu Q and Zhu X. Association of HLA-B27 status
and gender with sacroiliitis in patients with ankylosing spondylitis. Pak. J.
Med. Sci., 2014; 30(1): 22–27.
12. Oostveen
J, Prevo R, Den Boer J, Van de Laar M. Early detection of sacroiliitis on
magnetic resonance imaging and subsequent development of sacroiliitis on plain
radiography. A prospective, longitudinal study. J. Rheumatol., 1999; 26:
1953–58.
13. Sieper
J. Spondyloarthropathies in 2010: new insights into therapy-TNF blockade and
beyond. Nat. Rev. Rheumatol., 2011; 7: 78–80.
14. Harrison TR. Harrison’s Principles of Internal
Medicine. 16th ed., McGraw-Hill Companies, Inc. USA: 2005: pp. 1993.
15. Kijlstra
A, Luyendijk L, Vandergaag R. Vankregten E, Linssen A, et al. IgG and IgA
immune response against klebsiella in HLA-B27-associated anterior uveitis. Br.
J. Ophthal., 1986; 70: 85-88.
16. Neumuller
J, Schwartz DWM, Dauber E. Evaluation of four monoclonal antibodies against
HLA-B27 for their reliability in HLA-B27 typing with flow cytometry (FC):
comparison with the classic microlymphocytotoxic test. Cytometry, 1996; 26: 209–15.
17. Smith SM, Laurie AD,
Potter HC, McGettigan BD. HLA-B27 real-time PCR using TaqMan-MGB sequence specific probes. NZ J. Med. Lab Sci., 2014; 68: 04-08.