INTRODUCTION- Now-a-day
numerous herbicides are used for the controlling of crop weeds. Most of the
herbicides available in the market are synthetic, which is one of the major
causes of water pollution. This is widely used to control weed and herbaceous
pests. But, it greatly affects the quality and quantity of food production. These synthetic herbicides are directly used in the
agricultural field and due to runoff water and soil erosion arrives at nearly
water bodies such as a river, ponds, lakes, etc [1]. This can result
in the accumulation of a large amount of herbicides in such water bodies. The
normal aquatic flora and fauna including the fishes are greatly affected to
change in the environment. The fishes are directly exposed to the aquatic
environment and accumulate various toxic compounds in organs. The toxic
chemicals easily penetrate the fish’s body by various routes such as direct
contact, respiration by gills and food. The feeding of poisoned insect and
other fishes is one of the secondary causes of exposure. They are adverse
effects on the normal function, growth, behaviour and physiology of the fishes
because of low degradability, high rate of accumulation inside the aquatic
fauna and long term persistence [2].
Glufosinate
ammonium is a highly effective herbicide used to control weeds in more than 100
crops in many countries worldwide. Farmers rely on Glufosinate-ammonium because
it ensures a high degree of crop safety, as it only affects the parts of the
plant where it is applied. Glufosinate ammonium was first brought to market in
1984. Today it is registered for use to control weeds in a variety of crops
worldwide, including soybeans, corn, canola and cotton, which have been
modified through genetic engineering to be tolerant to Glufosinate-ammonium.
Proteins are a
fundamental biochemical component and available in huge amount in fishes.
Fishes have an important role in the diet because of the rich protein source
and their socioeconomic role for humans [3]. Analysis of protein
content in fishes helps to analyze healthy growth and nutritional value. The
study of protein content in the fish body used to be understood under the
stressful conditions and ability of fishes to overcome the toxic effects of
toxicants, metabolic activity, movement and during spawning. Therefore, the
present study was undertaken to evaluate the effect of lethal concentrations of
sweep power (Glufosinate Ammonium) on total protein content in fingerlings of
freshwater fish L. rohita.
MATERIALS
AND METHODS- The present study was conducted in the
Department of Zoology, Gopal Krishna Gokhale College Kolhapur, India from June
2019 to December 2020.
Experimental fish and laboratory
condition- The fingerlings of freshwater fish L. rohita (6±8 cm in length and 9-11 g
in weight) were collected from the local supplier from Kolhapur. Before the
acclimatization in laboratory condition, fingerlings were offering a bath with
a disinfectant solution to avert bruise and disease. After disinfection fish
were maintained in glass aquaria at 26±20C temperature, 12 hours
light and 12 hours dark cycle with supplying continuous aeration for 15 days.
The fishes were fed every day with commercially available fish food.
Toxicity Test- The
insecticide sweep power (Glufosinate Ammonium) was purchased from M/S Super Bio
Tech Marketing Company, India. A well-acclimatized healthy fishes were selected
for the present study. The toxicity test was carried out in 20 liter plastic
trough. In each trough, ten fishes were released. The trough was distinguished
into three groups viz. Control group (Without any exposure of toxicant); LC0
Concentration group (exposed to 0.01 ppm concentration of sweep power)
and LC50 concentration group (exposed to 0.05 ppm concentration of
sweep power). After 24hrs, the experimental medium
was replaced by a fresh medium.
Estimation of Total protein
content- After 96 hours of exposures, the fishes from each
group were sacrificed and total protein content was estimated by the Lowry
method [4] from gill, liver, muscle and brain tissues.
Statistical analysis- The
observed data from each group were expressed in arithmetic mean±standard
deviation. The level of significance was calculated using the student's t-test.
Ethical
approval- All the experimental procedures were carried out
with due permission of the Institutional Animal Ethics Committee, Gopal Krishna
Gokhale College Kolhapur, India.
RESULTS- The result of the effect of sweep
power (Glufosinate Ammonium) on the total protein in various organs viz. gill,
muscle, liver, brain of the fish L.
rohita in the control group, LC0 concentration group and LC50
concentration group after acute exposure (96 hours) are depicted in Table 1 and
Fig. 1.
Table 1:
Total protein content in different tissues of the fish L. rohita after acute exposure sweep power (Glufosinate ammonium)
|
Groups |
Amount of total protein (µg
protein/mg wet wt. of tissue) |
|||
|
Gill |
Liver |
Muscle |
Brain |
|
|
Control |
24.1±0.37 |
39±0.28 |
38.7±0.33 |
29.9±0.37 |
|
LC₀ |
21±0.28*** |
32.67±0.33*** |
36.1±0.37*** |
26.97±0.29*** |
|
LC₅₀ |
13.6±1.23*** |
31.23±0.46*** |
23.63±0.29*** |
18.07±0.43*** |
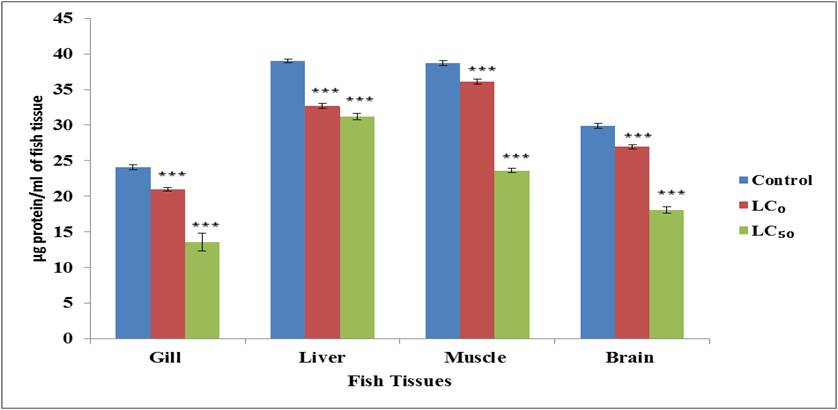
Fig. 1: Total
protein content in different tissues of the fish L. rohita after acute exposure sweep power (Glufosinate ammonium);
Values expressed as Arithmetic Mean of (n=5)±SD, ***=P<0.001
DISCUSSION- In the present study, observed the
toxicological effect of the agrochemical
herbicide Sweep Power (Glufosinate Ammonium) using total protein in the
freshwater fish L. rohita. Sweep
Power is the most recently used herbicides practised in many agriculture and
industrial fields. The major component of Sweep Power herbicide is Glufosinate
Ammonium. The Glufosinate Ammonium containing herbicides are also used as
a pre-harvest desiccant for a variety of crops including potatoes, peas,
soybeans and cereals [5]. Nowadays, it is also
increasingly being used as a selective herbicides plant on transgenic crops [6,7]. The Glufosinate Ammonium acts by blocking the enzymes
involved in the synthesis of amino acid glutamine. Inhibition of
glutamate synthesis has been reported not only in plants but also in animals
and humans [5,8]. Watanabe and Iwase [9] were reported the
toxic effect of Glufosinate Ammonium on mammalian brain cells both in vivo and
in vitro, in response to the release of excessive glutamic acid. Recent studies
have been observed the exposure of Glufosinate Ammonium leads to the
accumulation of free radicals and increased lipid peroxidation [10].
Due to runoff water, Glufosinate Ammonium, which
is excessively used in agriculture field reaches natural water recourses, which
harms non-target organism such as larvae of clams, oysters, daphnia, and
several species of freshwater fish [11-14].
An excessive amount of Glufosinate Ammonium has
been reported in agricultural soil, groundwater and stable water [15,16].
In the present
investigation, the amount of total protein content was significantly decreased
in all tissues (gills, liver, muscle and brain) of fish L.
rohita in all experimental groups (LC0
and LC50 concentration group) as compared to the control group. Similar
results were noted by various toxicity studies in fishes. Veeraiah et al. [17] revealed a
significant decrease in total protein in freshwater fish L. rohita exposed to acute and chronic concentrations of pesticide
Indoxacarb (LC50=0.0531). Lekeshmanaswamy [18] reported
that the sub-lethal dose of malathion in the exposed freshwater fish L. rohita showed a decline in protein
content. Decreased levels of protein in gills, liver, brain and muscle of Cirrhinus mrigala under the acute concentration
of methanol reported by Desai and Bhilave [19]. Prakash and Verma [20]
observed a gradual decrease in protein content in the liver and muscles of
catfish (Mystus vittatus) exposed to
sub-lethal concentration of arsenic.
In the
present study, the decrease in total protein content in various tissues of fish L. rohita is
might be due to increased stressful conditions by acute intoxication of
selected herbicide [21]. To cope with this insecticides stress,
fishes demand more energy and this result in increased proteolysis [22]. Chandravathy
and Reddy [23] reported the decreased total protein content in
muscle tissue is due to inhibition of protein synthesis, elevated protein
degradation and increased protein utilization for various metabolic reactions
under stress. Reduction in the protein content in tissue is due to increased
proteolysis and utilization of products for various metabolic reactions. Due to
insecticide toxicity, there may be increased necrosis of tissues, damage of
cellular membranes, cytoplasmic vacuolation, cellular degradations, etc. This
can result in the depletion of protein owing to the utilization of protein for
cell repair system [24].
CONCLUSIONS-
A
decrease in the total protein level in the present investigation in L. rohita indicated that the gill, liver, muscle and brain tissue protein
might have proteolysis activity, stressful condition, increased catabolic
reaction and protein synthesis to fulfil increased energy demand during the
acute toxic condition of herbicide Sweep Power (Glufosinate
Ammonium).
Further studies
were required for the effect of Glufosinate Ammonium at the molecular level in
freshwater fish L.
rohita and other
non-target animals.
CONTRIBUTION OF AUTHORS
Research concept- Dr.
Manjiri A. More, Dr. Narayan R. Mane
Research design-
Dr. Manjiri A. More, Dr. Narayan R. Mane
Supervision-
Dr. Manjiri A. More, Dr. Narayan R. Mane
Materials-
Dr. Manjiri A. More, Dr. Narayan R. Mane
Data collection-
Dr. Manjiri A. More, Dr. Narayan R. Mane
Data analysis and
interpretation- Dr. Manjiri A. More, Dr. Narayan R. Mane
Literature search- Dr.
Manjiri A. More, Dr. Narayan R. Mane
Writing article-
Dr. Manjiri A. More, Dr. Narayan R. Mane
Critical review-
Dr. Manjiri A. More, Dr. Narayan R. Mane
Article editing-
Dr. Manjiri A. More.
Final
approval- Dr. Manjiri A. More, Dr. Narayan R. Mane
REFERENCES
1.
Liu W, Gan JJ, Lee S, Kabashima JN.
Phase distribution of synthetic pyrethroids in runoff and stream water. Environ
Toxicol Chem., 2004; (1): 7-11.
2.
Mintram KS, Maynard SK, Brown AR, Boyd
R, Johnston AS, et al. Applying a mechanistic model to predict interacting
effects of chemical exposure and food availability on fish populations. Aquat
Toxicol., 2020; 224: 105483.
3.
Massey LK. Dietary animal and plant
protein and human bone health: a whole foods approach. J Nutr., 2000; 133(3):
862S-5S.
4.
Lowry OH, Rosenber NJ, Farr AL, Randal
RJ. Protein measurement with Folin phenol reagent. J Biochem., 1951; 193:
265-75.
5.
Kang GR, Song HY, Kim DS. Toxicity and
effects of the herbicide glufosinate-ammonium (Basta) on the marine medaka Oryzias dancena. J Fish Aquat Sci.,
2014; 17(1): 105-13.
6.
Duke SO, Cerdeira AL. Transgenic crops
for herbicide resistance. In Transgenic crop plants. Springer, Berlin,
Heidelberg, 2010; pp. 133-66.
7.
Andreassen ÅK, Bakke AM, Dahl KK, Dalen
KT, Finne MA, et al. Final Health and Environmental Risk Assessment of
Genetically Modified Soybean A5547-127. Eur J Nutr Food Saf.,
2021: 130-31.
8.
Cox C. Herbicide factsheet: glufosinate.
Journal of pesticide reform: a publication of the Northwest Coalition for
Alternatives to Pesticides (USA), 1996.
9.
Watanabe T, Iwase T. Developmental and
dysmorphogenic effects of glufosinate ammonium on mouse embryos in culture.
Teratog carcinog mutagen, 1996; 16(6): 287-99.
10.
Takano HK, Dayan FE. Glufosinate‐ammonium:
a review of the current state of knowledge. Pest Manag. Sci., 2020; 76(12): 3911-25.
11.
Qian H, Chen W, Sheng GD, Xu X, Liu W,
et al. Effects of glufosinate on antioxidant enzymes, subcellular structure,
and gene expression in the unicellular green alga Chlorella vulgaris. Aquat Toxicol., 2008; 88(4): 301-07.
12.
Peltzer PM, Junges CM, Attademo AM,
Bassó A, Grenón P, Lajmanovich RC. Cholinesterase activities and behavioral
changes in Hypsiboas pulchellus
(Anura: Hylidae) tadpoles exposed to glufosinate ammonium herbicide.
Ecotoxicol., 2013; 22(7): 1165-73.
13.
Lajmanovich RC, Cabagna-Zenklusen MC,
Attademo AM, Junges CM, Peltzer PM, Bassó A, Lorenzatti E. Induction of
micronuclei and nuclear abnormalities in tadpoles of the common toad (Rhinella arenarum) treated with the
herbicides Liberty and glufosinate-ammonium. Mutat Res Gen Tox En., 2014; 769:
7-12.
14.
Xiong G, Deng Y, Li J, Cao Z, Liao X,
Liu Y, Lu H. Immunotoxicity and transcriptome analysis of zebrafish embryos in
response to glufosinate-ammonium exposure. Chemosphere, 2019; 236:124423.
15.
Ferrari S, Mettifogo OS, Cunha ML, dos
Santos Cordeiro LF, do Valle Polycarpo G, et al. Effects of Low Doses of
Glufosinate-Ammonium on Upland Rice Agronomic Traits. Gesunde Pflanzen., 2021:
1.
16.
Geng Y, Jiang L, Zhang D, Liu B, Zhang
J, et al. Glyphosate, aminomethylphosphonic acid, and glufosinate ammonium in
agricultural groundwater and surface water in China from 2017 to 2018:
Occurrence, main drivers, and environmental risk assessment. Sci Total Environ., 2021; 769: 144396.
17.
Veeraiah K, Padmavathi P, Rao ST, Vivek
CH. Methyl parathion (50% EC) induced changes in protein and DNA banding
patterns in the fish Channa punctatus
(Bloch). Int J Bioassays, 2014; 4: 3632.
18.
Lekeshmanaswamy M. studies on the impact
of a malathion insecticide on certain biochemical constituents of a freshwater
fish, Labeo rohita. Kong. Res. J.,
2018; 5(1): 93-96.
19.
Desai T,
Bhilave M. Toxicological Effect of Methanol on Protein Profile of Freshwater
fish Cirrhinus mrigala. IJRA, 2019;
6(2): 309-20.
20.
Prakash SA, Verma AK. Impact of Arsenic
on Protein Metabolism of a fresh water cat fish, Mystus vittatus. UPJOZ, 2020; 41(5): 16-19.
21.
Somaiah K, Satish PV, Sunita K, Nagaraju
B, Oyebola OO. Toxic impact of phenthoate on protein and glycogen levels in
certain tissues of Indian major carp Labeo
rohita (Hamilton). IOSR J Environ Sci Toxicol Food Technol., 2014; 8:
65-73.
22.
Pazhanisamy K, Indra N. Toxic effects of
arsenic on protein content in the fish, Labeo
rohita (Hamilton). Nat Environ
Pollut Technol., 2007; 6(1): 113-16.
23.
Chandravathy VM, Reddy SL. In vivo recovery of protein metabolism
in gill and brain of a freshwater fish, Anabas
scandens after exposure to lead nitrate. J Environ Biol., 1994; 15(1):
75-82.
24.
Lecker SH, Goldberg AL, Mitch WE.
Protein degradation by the ubiquitin–proteasome pathway in normal and disea